Who knew there was so much science in a simple bubble?
We’ve all blown bubbles before, but do you know about the science of bubbles? How can we make our bubbles better, stronger, and even more awesome?
Gather up your family and friends for some bubble blowing!

Let’s Make OMSI’s Bubbles!
Materials
1 Cup water
2 Tablespoons dish soap (Dawn is great)
1 Tablespoon glycerin
1 Tablespoon white corn syrup
Activity Instructions
Mix up your bubble solution
Use OMSI’s favorite recipe or make your own.
Blow some bubbles!
Use bubble wands from other bubble kits or make your own with pipe cleaners, string, or even your own hands!
Make some observations of your bubbles
Can you catch a bubble on your bubble wand? What do you see when you look at your bubble closely?
What did you observe?
Let’s Think…
How is a bubble like a ball? How is a bubble different than a ball?
Why do bubbles pop so easily?
If you blow a bubble from a triangle shaped wand, what shape will the bubble be?
Can you catch a bubble with your hand? Why or why not?
Now that you are a bubble pro, try some of these challenges!
What is the biggest bubble you can make?
Can you blow a bubble inside another bubble?
How long can you make one bubble last?
Can we make our bubbles stronger?
Try adding a little baking powder, gelatin, corn starch, or guar gum to your bubble solution.
What changed?
Can you make a bubble in a different shape?
Use straws and pipe cleaners to make bubbles shaped like pyramids, cubes, and more!
What’s the Science?
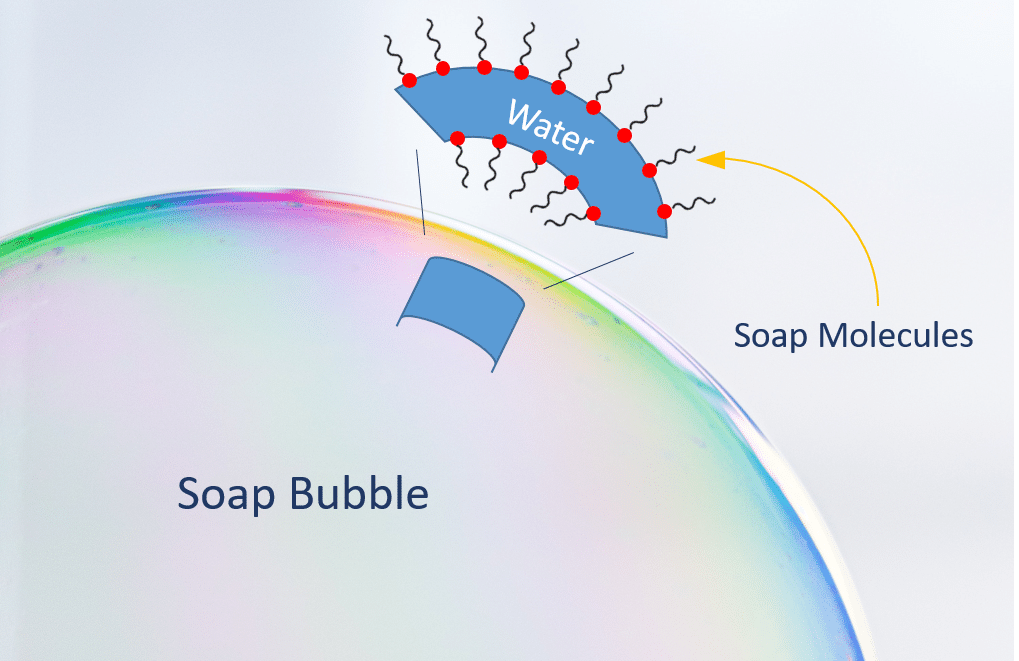
Bubbles are made up of two kinds of molecules. Water molecules and soap molecules. We need both together for bubbles to form.
To really understand how bubbles form, we need to understand surface tension. Surface tension is how well molecules can hold onto the others next to them. When water molecules are by themselves, they can’t form bubbles because they’re holding onto their neighbors too closely. Soap molecules are special in that they have two ends – one end that loves water and one end that very much does not.
When we combine soap and water together, those molecules get all mixed up together. This lessens the surface tension to make the liquid more stretchy. When we blow through a bubble wand that’s dipped in our bubble juice, we’re stretching that mixture around air!
Anatomy of a Bubble – Molecules make it happen!
What makes bubbles pop?
Now we know that bubbles are held together by the surface tension of the water that makes them. One reason a bubble might pop is if it gets poked. When we poke a bubble, we form a hole in that surface tension and the rest of the bubble shrinks away and breaks apart.
The second reason a bubble might pop is evaporation. If the water in the bubble evaporates away into a gas, there won’t be any more water to help hold the bubble together and the bubble pops.
More Ideas
Bubble Fandom
If you are loving bubbles more than you ever expected, check out this Soap Bubble Wiki!
TED Talk On Bubbles
Still curious and want to hear more about bubbles? Check out this TED Talk!
Explore All!
Check out all of OMSI’s Science at Home videos and experiments.
